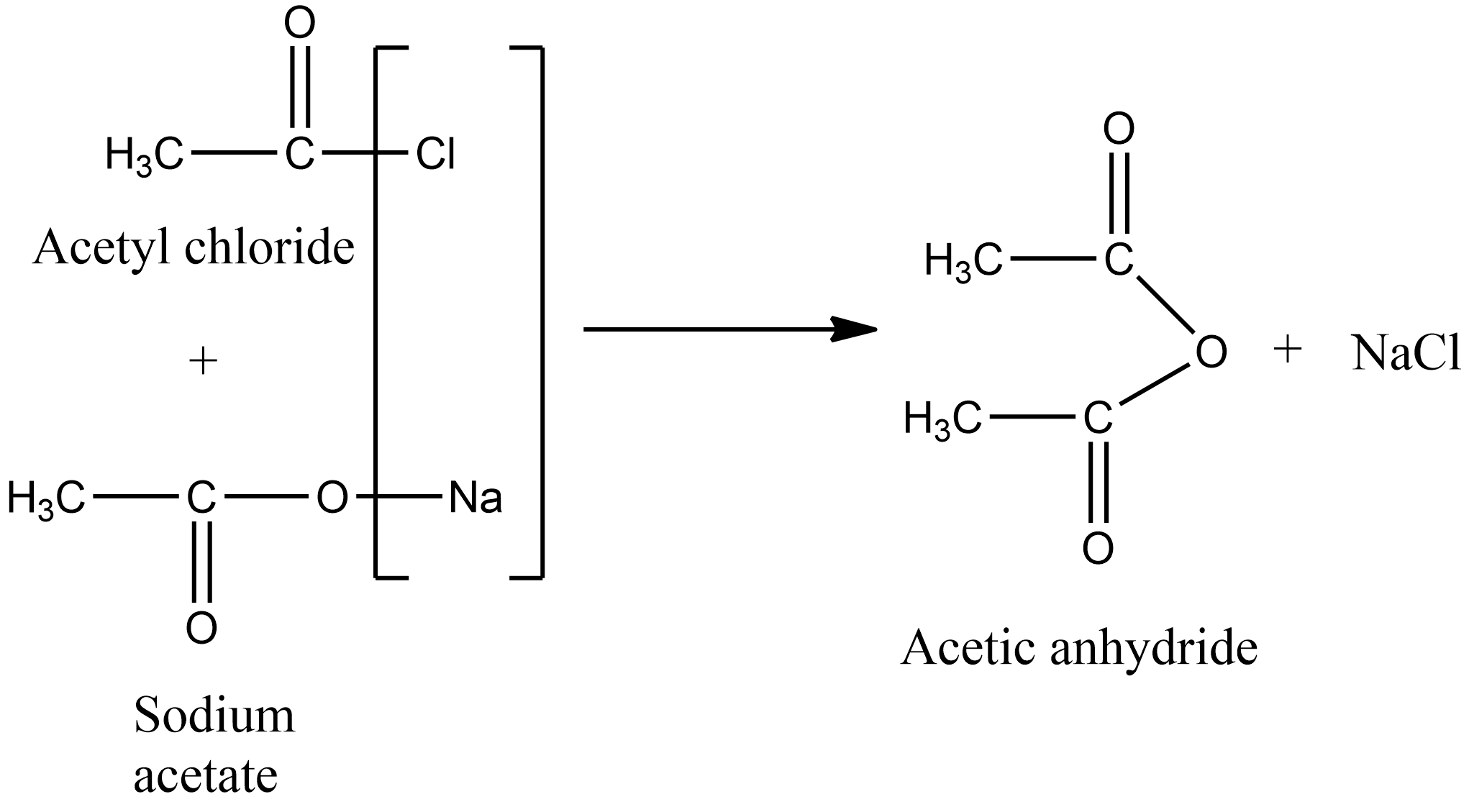
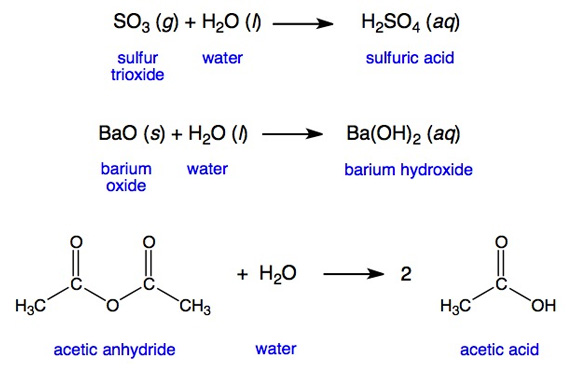
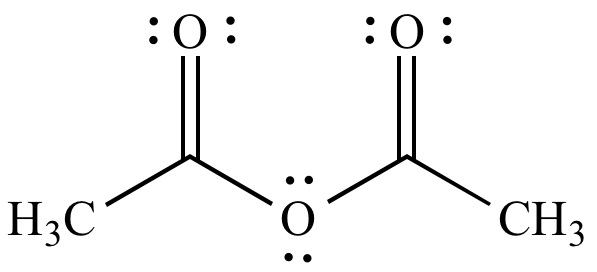
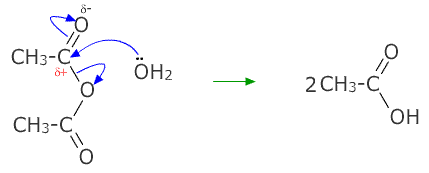
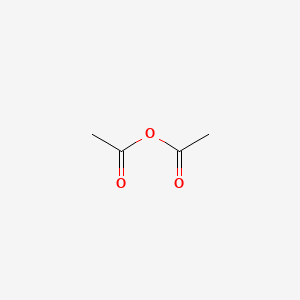
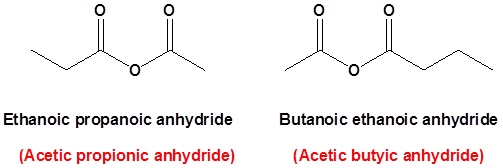
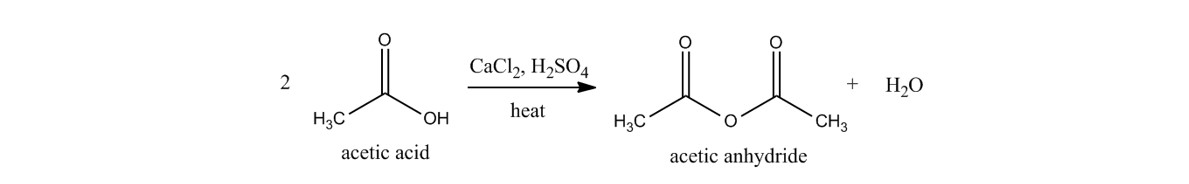
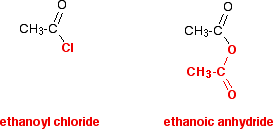
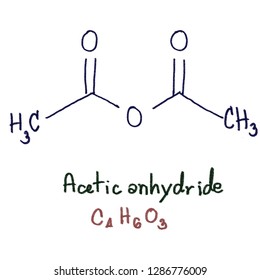
acetic anhydride - 108-24-7, C4H6O3, density, melting point, boiling point, structural formula, synthesis

organic chemistry - Mechanism for Synthesis of Tetranitromethane from Acetic Anhydride and Fuming Nitric Acid - Chemistry Stack Exchange

Scheme 1. Reagents and conditions: (i) acetic acid anhydride, reflux, 1... | Download Scientific Diagram

a) The name acetic anhydride implies that the compound will react with water to form acetic acid. Write the equation for the reaction. b) Write the equation for the reaction by which

What is the Difference Between Acetic Acid and Acetic Anhydride | Compare the Difference Between Similar Terms
Acetic acid anhydride, 10 l, plastic | Reagents for Peptide Synthesis for Washing/Deprotection/Splitting | Peptide Synthesis | Organic & Bioorganic Chemicals | Chemicals | Carl Roth - International