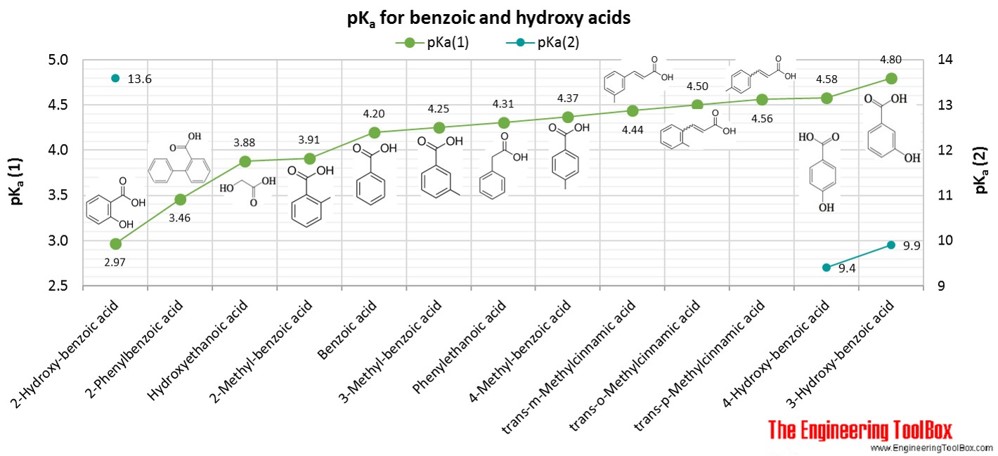
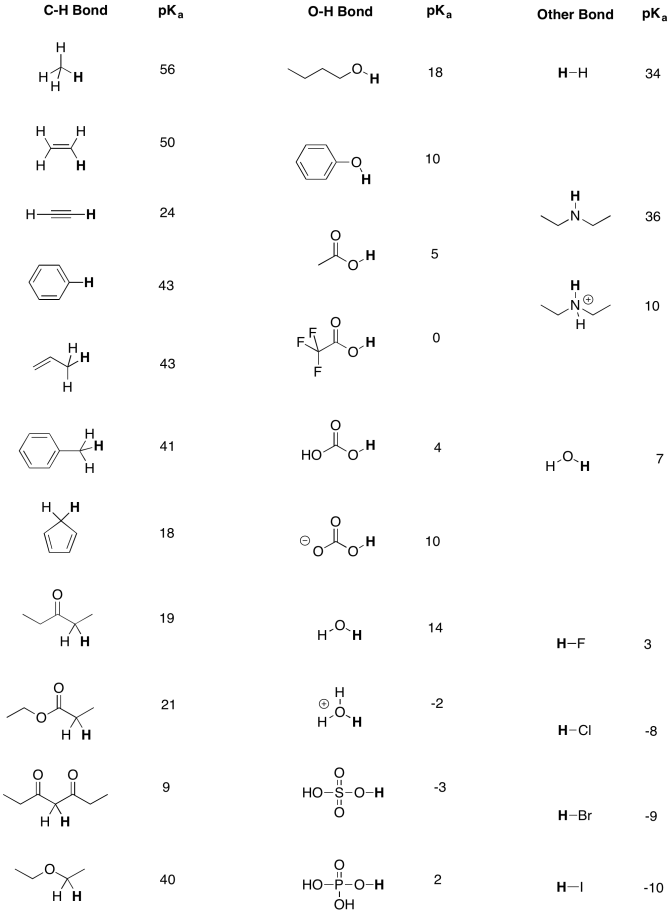
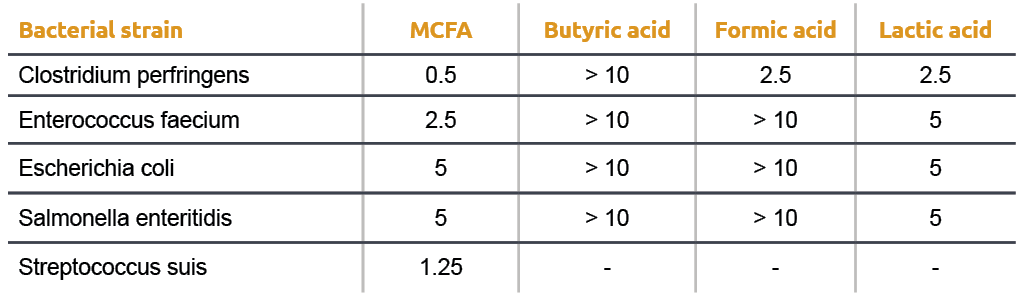
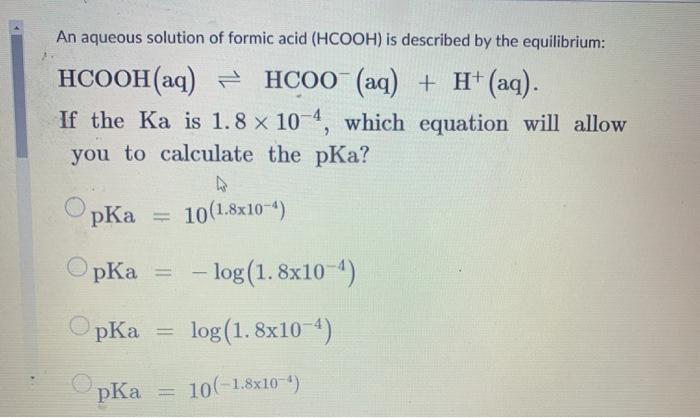
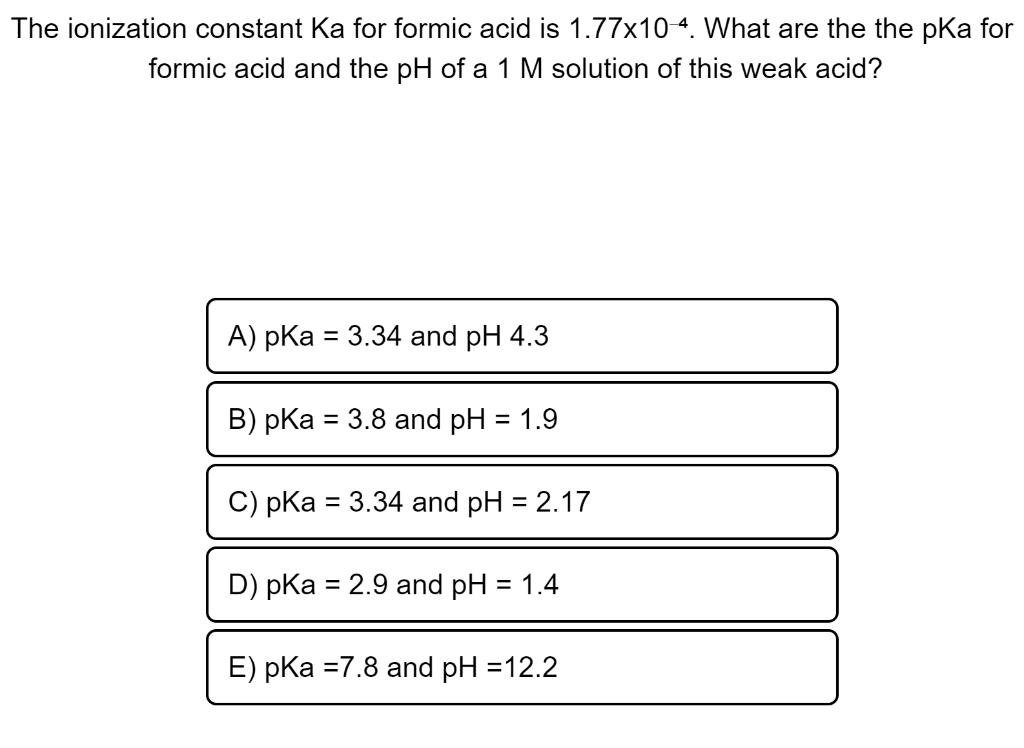
If you have 500 mL of 0.15 M formic acid, what is the pH of this solution? What is the pKa? How many grams of sodium formate would you have to add

The comparison of pKa determination between carbonic acid and formic acid and its application to prediction of the hydration numbers - ScienceDirect

If you have 500 mL of 0.15 M formic acid, what is the pH of this solution? What is the pKa? How many grams of sodium formate would you have to add
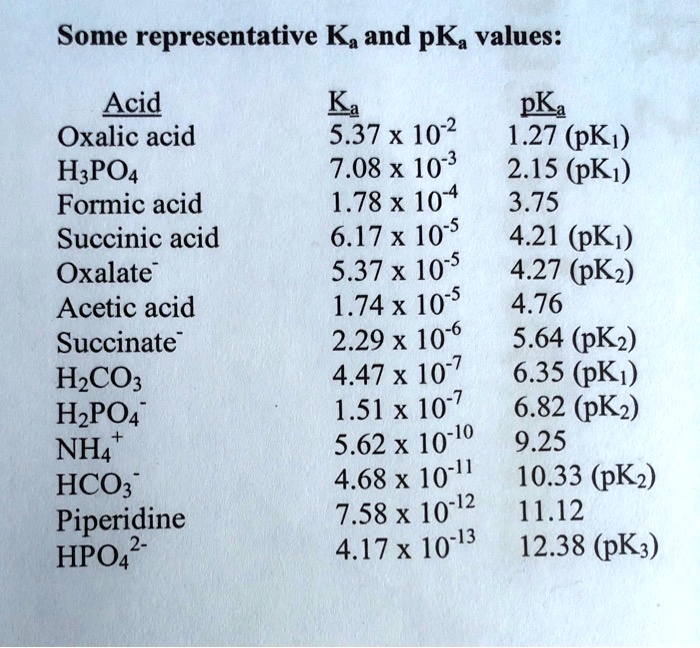
SOLVED: Some representative Ka and pKa values: Acid Oxalic acid HzPOa Formic acid Succinic acid Oxalate Acetic acid Succinate HzCOz HzPOA NH4' HCO; Piperidine HPOA2 Ka pKa 5.37 x 10-2 1.27 (pK,)

What is the pH of a 0.15 M solution of formic acid, HCOOH ? `{:("Formic Acid ",K_a),(HCOOH - YouTube

Value of dissociation constant of acetic acid is 10^-6 , where as dissociation constant of formic acid is 10^-5 . Which of the following will be the value of pKa (acetic acid) - pKa (formic acid)?

OneClass: The pKa of formic acid is 3.75. What is the buffering range (buffering region) of a buffer ...

Experimental pKa values and structures of the conformers of acetic,... | Download Scientific Diagram