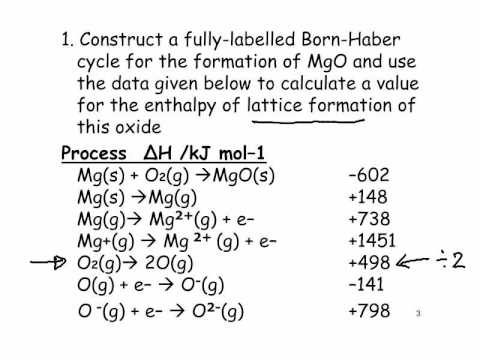
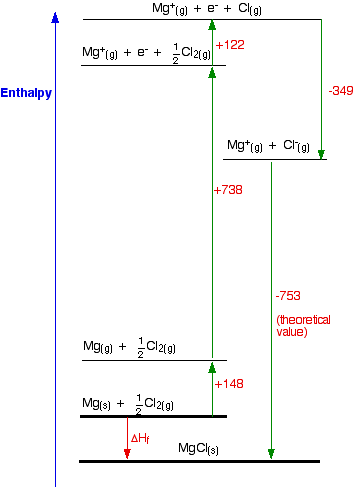
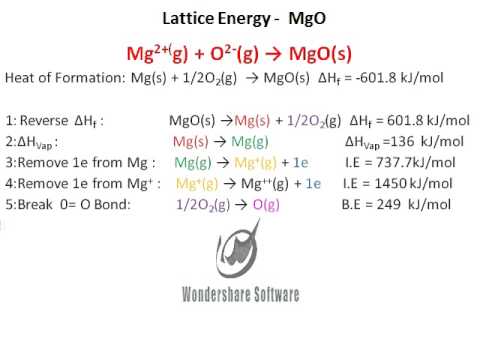
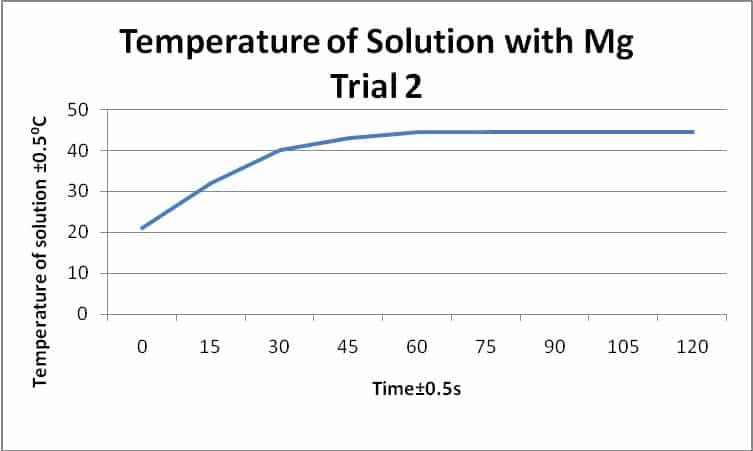
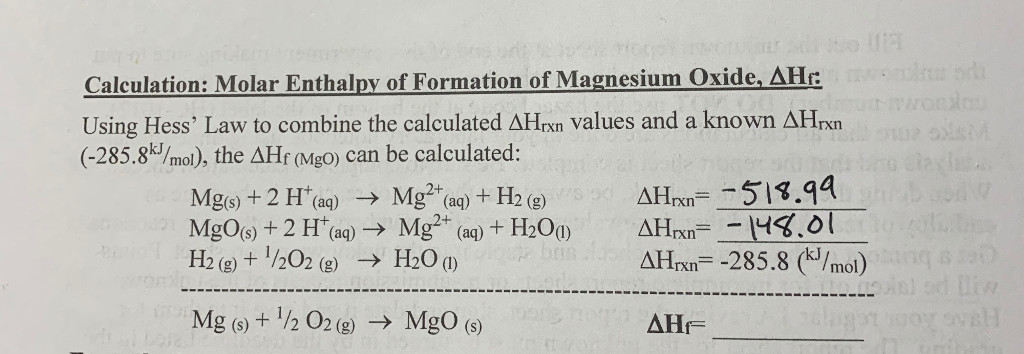
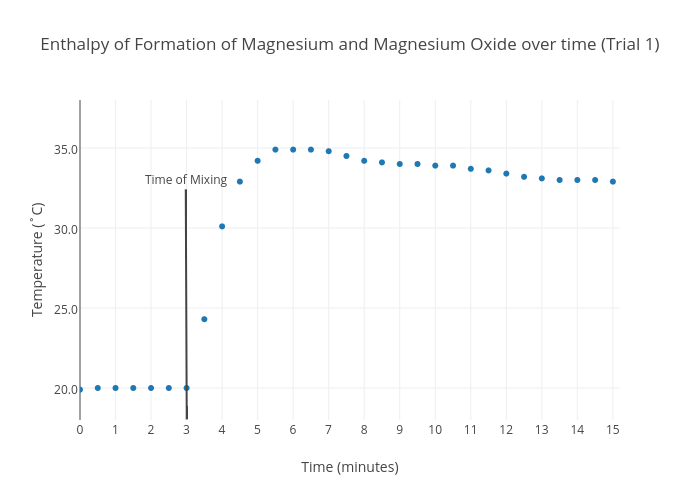
Hess's Law. The experiment conducted was meant to determine the enthalpy of formation of MgO(s) and CaO(s) - International Baccalaureate Chemistry - Marked by Teachers.com

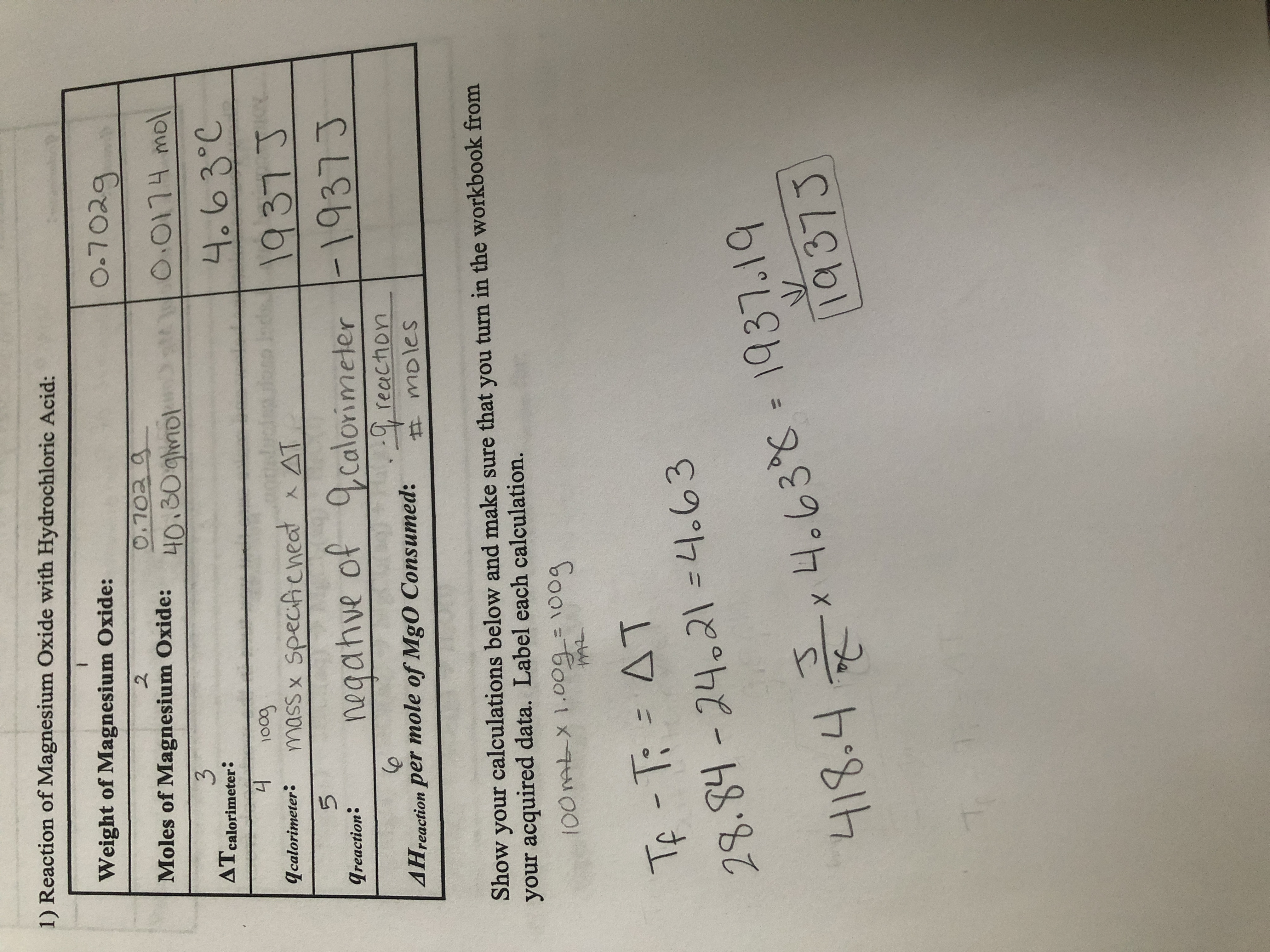
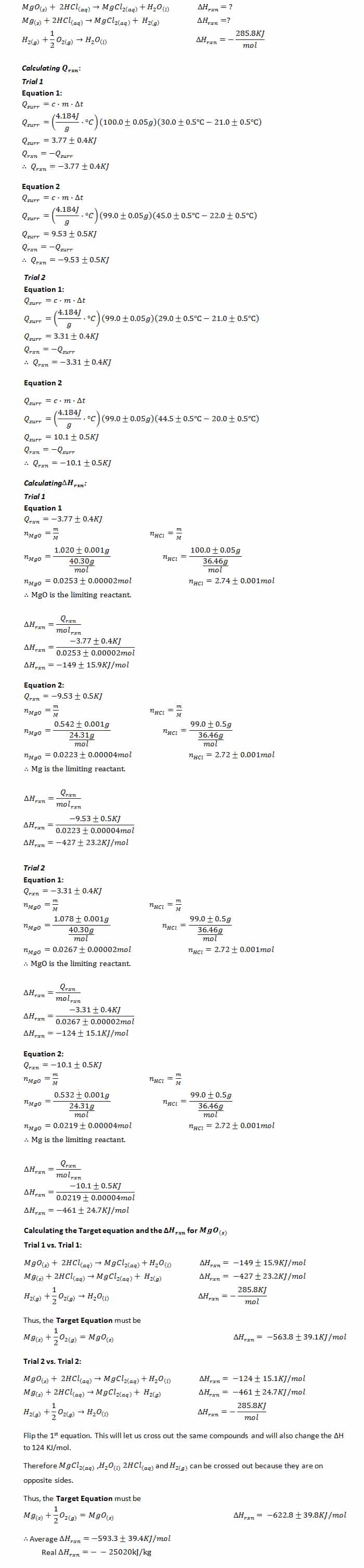
Instructions - Lab 4 - Enthalpy of Formation of Mg O (PDF Instructions + Graph Paper) - CHEM 115: - Studocu
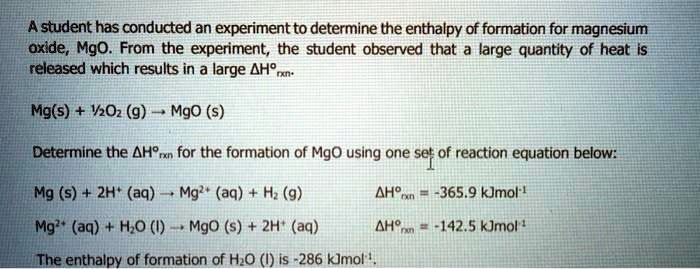
SOLVED: Astudent has conducted an experiment to determine the enthalpy of formation for magnesium oxide, Mgo. From the experiment; the student observed that large quantity of heat Is released which results in
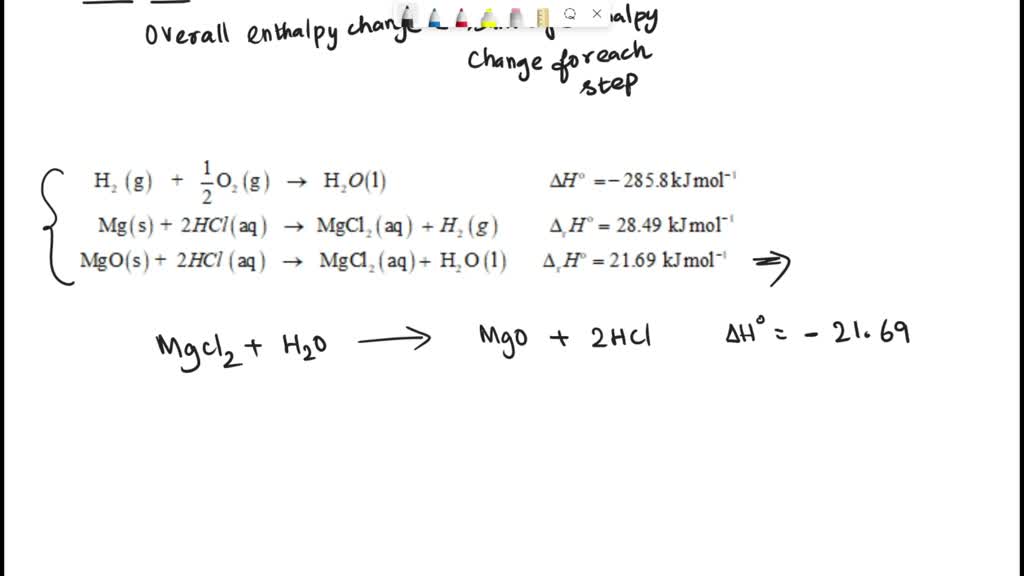
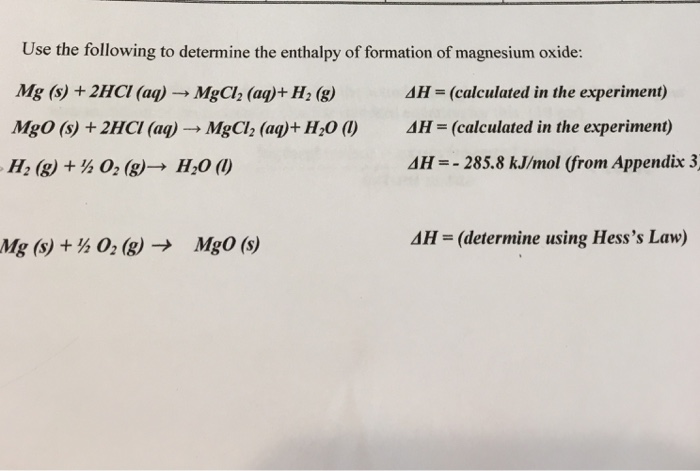
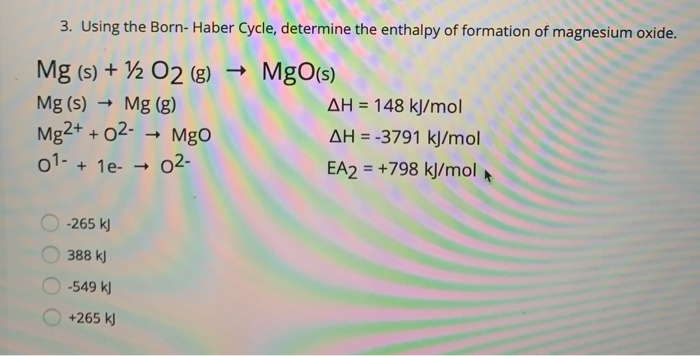
SOLVED: determine the enthalpy of formation You can use your experimental data and Hess' Law to thermochemical equation: ofMgon The enthalpy of formation of Mgo is given by this Mgc) Yh0z(r) MgOt)

Enthalpy of Formation of Magnesium Oxide - Experiment 9: Enthalpy of Formation of Magnesium Oxide - Studocu
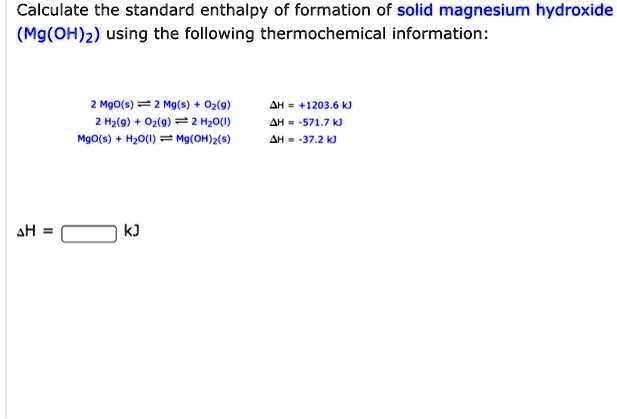
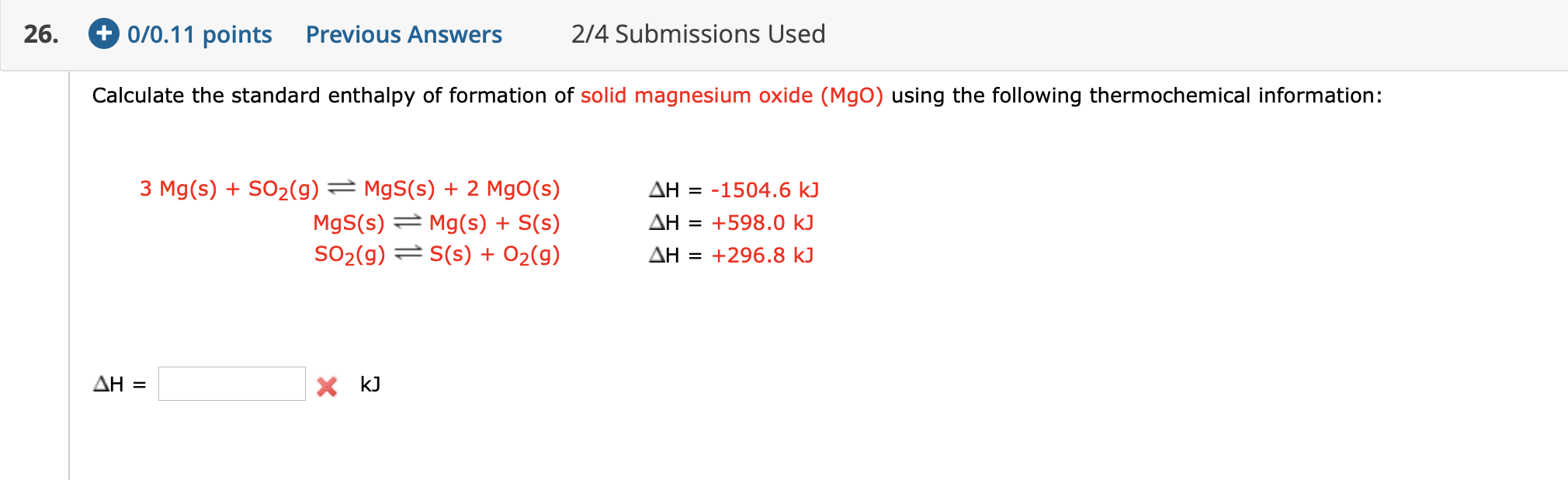
SOLVED: Calculate the standard enthalpy of formation of solid magnesium hydroxide (Mg(OH)z) using the following thermochemical information: MQoxs) = 2 Mg(s) 0z(9) Hz(9) 02(9) = Hzo(u) MqO(s) H,o() = Mg(Oh)z(s) 71203.6 kJ